Het Anabolenboek
Willem Koert
Aede de Groot
Wageningen, 12/4/2007
11. Metabolism of Testosterone, Dihydrotestosterone, Estrone and Estradiol
Aede de Groot, Willem Koert
Testosterone is the most important androgenic-anabolic steroid. It is biosynthesized in the testes and per day about 4-10 mg of testosterone is produced by a grown up man. About 0.5 mg per day is produced in the adrenaline cortex of men and women.
Testosterone is converted to two other important hormones. It is reduced to dihydrotestosterone in specific tissues like the skin and the prostate and it is oxidized to estradiol. In men this oxidation mainly takes place in adipose tissue and in the testes. In women the biosynthesis of estradiol takes place in the ovaria.
Testosterone and dihydrotestosterone together are responsible for the typically male sex characteristics, but their function is different. In the adolescence testosterone induces the sex drive in men, enlargement of the penis, the production of sperm, increase of muscle mass and lowering of the voice, the so called anabolic effects.
Dihydrotestosterone is responsible for an increase of body hair, beard grow, acne, and at a later age for baldness and enlargement of the prostate. These are the androgenic effects.
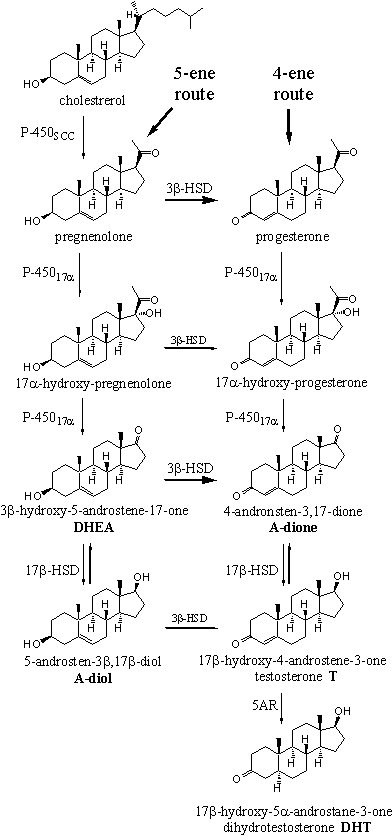
Much research has been devoted to unravel the biosynthesis of steroid hormones, because promising medical applications were expected. Many reviews have appeared in the literature about the biosynthetis of the male and female sex hormones [1][2]. The main features of the biosynthetic pathways of testosterone, dihydrotestosterone, estrone and estradiol are know today as they are depicted in Schemes 1 and 2.
The biosynthesis of the sex hormones starts with the oxidation of the side chain of cholesterol, which is catalyzed by the enzyme cytochrome P450scc. This cytochrome P450 oxidizes the side chain on C20 and C22 by the introduction of two hydroxyl groups. After that the chain is broken in between these two atoms by the same enzyme, under formation of pregnenolone.
The next steps in the biosynthesis of testosterone can proceed via two different routes. Pregnenolone can be oxidised first by cytochrome P45017a to 17a-hydroxypregnenolone. This route is known as the 5-ene route because all biosynthetic intermediates in this route possess a D5-double bond.
The enzyme 3b-HSD also can convert pregnenolone first into progesterone by oxidation of the 3b-hydroxy group followed by a shift of the double bond from the C5-C6 to the C4-C5 position. This route is known as the 4-ene route because here all biosynthetic intermediates possess a D4-double bond.
Both pregnenolone and progesterone are accepted as substrate by the enzyme cytochrome P45017a. In both compounds a hydroxyl group is introduced in the 17a-position, as is indicated in the name of the enzyme. The rupture of the C17-C20 bond is catalyzed by the same enzyme. This results in the complete removal of the side chain under formation of a carbonyl group at C17. In this way 4-androstene-3,17-dione (A-dione) is obtained via the 4-ene route, and 3b-hydroxy-5-androstene-17-one (DHEA) via de 5-ene route. The acronyme DHEA originates from the old-fashioned name dehydroepiandrosterone for this compound. DHEA has a weak anabolic activity from itself.
Scheme 1
The oxidation of the 3b-hydroxyl group and the shift of the D5-double bond to the D4-position, are also catalyzed by one enzyme, called 3b-hydroxy-steroid dehydrogenase/D5-D4-isomerase, or 3b-HSD.
The enzyme 3b-HSD first oxidizes the hydroxyl group at C3 to a carbonyl group and after that the same enzyme catalyzes the isomerisation of the double bond to the D4-position. This oxidation-isomerisation reaction proceeds in only one direction. In principle this enzyme accepts all compounds in the left column of Scheme 1 as a substrate but pregnenolone and DHEA are the main substrates. This is indicated in Scheme 1 with bold arrows.
The transformation of A-dione into testosterone now only needs the reduction of the C17 carbonyl group to a 17b-hydroxyl group. This reaction is catalysed again by a dehydrogenase enzyme, called 17b-hydroxy-steroid dehydrogenase or 17b-hydroxysteroid oxidoreductase or simply 17b-HSD. This enzyme adds two H-atoms to the carbonyl group, one to the O-atom and the other one to the bottom of C17, which results in the b-position of the hydroxyl group.
In several tissues testosterone is converted into 5a-dihydrotestosterone (DHT) by the enzyme 3-oxo-a-steroid-D4-dehydrogenase, or 5AR. The indication “5a-steroid-D4 ” in the name of this enzyme shows that the H-atom is delivered to the bottom, the a-side, of the molecule of the D4-double bond, as is indicated in the structural formula of dihydrotestosterone by a hatched bond. Also this reaction proceeds in only one direction.
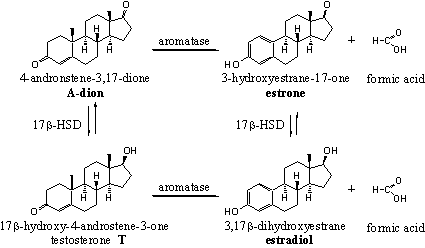
The estrogens estrone and estradiol are, together with progesterone, the most important female sex hormones. The estrogens are responsible for the typical female sex features, such as the growth and development of the vagina, the uterus, the Fallopian tubes, the development of breasts and the increase of fat tissue. Together with progesterone they regulate the menstruation cycle. The activity of estradiol is much larger than that of estrone. Both compounds can be interconverted by the enzyme 17b-HSD.
Scheme 2
The biosynthesis of the female sex hormones starts from A-dione and can proceed by two different routes (see Scheme 2). The C17 carbonyl group in A-dione can be reduced first to a 17b-hydroxyl group under formation of testosterone, followed by oxidation and removal of the C19 methyl group and aromatisation of ring A.
The oxidation and removal of the C19 methyl group and the aromatisation of ring A can take place also first, under formation of estrone. Reduction of the carbonyl group at C17 then gives estradiol.
The oxidation of the C19 methyl group is catalyzed by a complex of cytochrome P450 enzymes, indicated with the code P450aromatase or simply as aromatase. The methyl group is first oxidized to a hydroxyl group and than to a carbonyl group (an aldehyde). The removal of this group together with a H-atom from C1 than leads to aromatisation of ring A. An important step in this aromatisation process is the fission of the C10-C19 bond. Also this reaction proceeds in only one direction. In chapter 15 more will be explained about aromatisation of anabolic steroids.
In schemes 1 and 2 we have seen that testosterone can be metabolized in two ways. In Scheme 1 the reductive metabolism leading to dihydrotestosterone and in Scheme 2 the oxidative metabolism to estradiol is shown. The reduction of testosterone takes place in target tissues like the prostate and the skin and of course metabolism takes place in the liver. In male a very small part (0.2%) of the testosterone is converted into estradiol. This process mainly takes place in adipose tissue and for about 20% in the testes.
The metabolisme of testosterone and dihydrotestosterone takes place for 90% in the liver. There reductases and dehydrogenases catalyse the reactions of the D4-double bond, the C3-carbonyl group and the C17-hydroxyl group. Finally the hydroxyl groups are connected to glucuronic acid or sulphate, followed by excretion with the urine [3] [4].
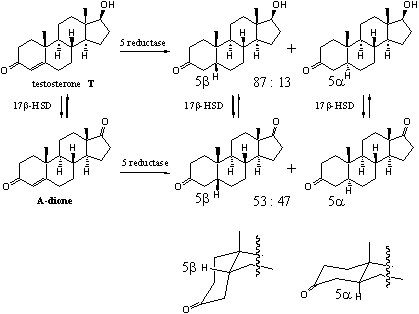
The reduction of the D4-double bond is not an equilibrium reaction and goes only in one direction to give a 5a and/or a 5b-steroid skeleton (see Scheme 3). The ezyme 5a-reductase is especially active in the prostate giving 5a-dihydrotestosterone. In the liver 5a- and 5b-reductases both are active and convert testosterone and other steroids into 5a- and 5bsteroid skeletons in ratios that depend on the structure of the steroid.
An example is the different ratio of 5a- and 5b-reduction products in testosterone and A-dione. The difference in their structures is that testosterone has a C17b-hydroxyl group and A-dione has a C17-carbonyl group. In testosterone the ratio between 5b : 5a-reduction products is 87 : 13. In A-dione this ratio is 53 : 47 (see Scheme 3) [3]. In other steroids this ratio can be completely different. There is mostly a preference for 5b-reduction.
Scheme 3
After reduction of the D4-double bond often a quick reduction of the C3-carbonyl group is observed. In the 5a-steroids there is a preference for reduction to the 3b-hydroxyl compounds. In the 5b-steroids mostly reduction to the 5a-hydroxyl compounds takes place.
The C3-and C17-dehydrogenases catalyse reversible reactions, thus reactions that can proceed in two directions. The C3-dehydrogenases can reduce the C3-carbonyl group to 3a- or 3b-hydroxyl groups, which both can be oxidized again to a carbonyl group. The C17-dehydrogenases oxidise the 17b-hydroxyl group to a carbonyl group, which can be reduced again to a 17b- or a 17a-hydroxyl group.
Finally a molecule of glucuronic acid is connected to the hydroxyl groups at C3, C17 or both by an enzyme called glucuronosyl transferase (UGT) [5]. About 10% of the hydroxyl groups are converted into sulfates by sulfatases. Both groups enhance the polarity of the whole molecule considerably and in this way the apolar steroids become soluble in water and can be excreted with the urine.
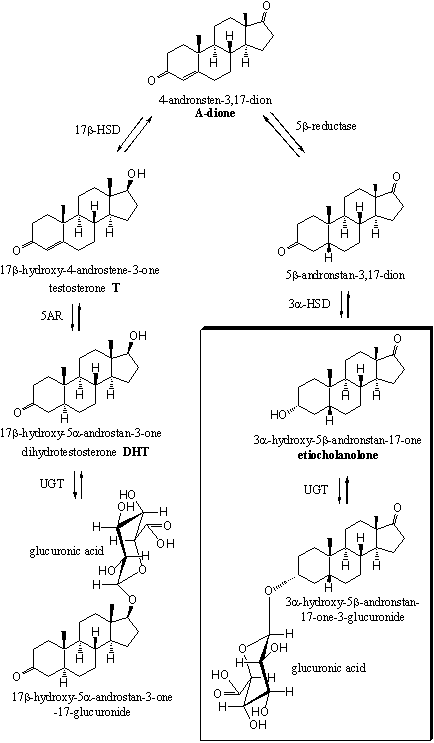
In Scemes 4 and 5 a number of the possible metabolic reactions of testosterone and dihydrotestosterone are depicted. The most important excretion products are 3a-hydroxy-5b-andronstane-17-one (also called etiocholanolone) and 3a-hydroxy-5a-androstane-17-one (also called androsterone). They are mostly excreted as glucuronides. These compounds are enclosed in a frame in schemes 4 and 5.
Scheme 4
The other possible metabolites have been found in the urine in only minor concentrations. These are 5a- or 5b-reduced steroids with 3a- or 3b-hydroxylgroups, 17 carbonyl groups and 17a- or 17b-hydroxyl groups. These compounds are depicted only partly in schemes 4 and 5.
The metabolisme of orally taken testosterone is fast. About 90% of the hormone is already metabolized before it reaches the bloodstream. After that the half-life of testosterone is less then 30 minutes, which means that the concentration halves within every half hour. It is therefore not a surprise that many researchers have investigated other ways of administration.
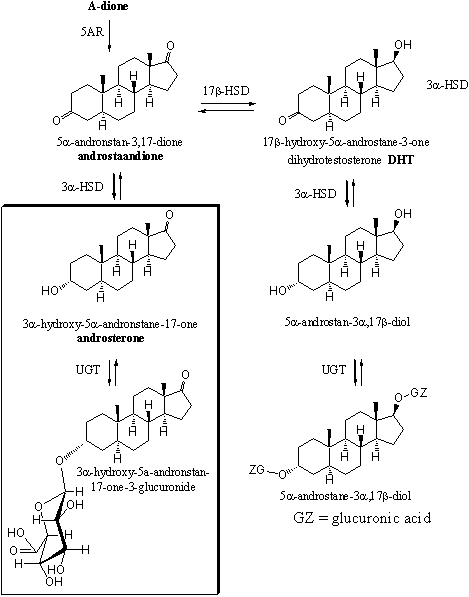
Scheme 5
In the case of testosterone solutions for this problem have been found in the use of esters. These derivatives are converted slowly into free testosterone by esterases. Other solutions are the application of plasters or crèmes which slowly release testosterone directly in the bloodstream. As soon as the synthetic testosterone reaches the blood, it is metabolised quickly again, which necessitates a constant supply.
Chemists also can change the structure of the steroid a bit so that metabolism is slowed down or prevented. These possibilities have been investigated extensively and have been directed toward the development of orally active anabolic steroids. Also enhanced activity and a better separation between anabolic and androgenic effects are still under investigation.
[1] Brown, G.D. Natural Product Reports (1998) 661-665.
[2] Brueggemeier R.W. Encyclopedia of Molecular Cell Biology and Molecular Medicine Vol 13, 1-69.
[3] Schanzer W. Clinical Chemistry (1996) 42, 1101-1020.
[4] Van Eenoo P., Delbeeke F.T. J. of Steroid Biochem. and Mol. Biochem. (2006) 101, 161-178.
[5] Belanger A., Pelletier G., Labrie F., Barbier O. and Chouinard S. Trends in Endocrin. and Metab. (2003) 14, 473-479.
|
|