Het Anabolenboek
Willem Koert
Aede de Groot
Wageningen, 18/10/2006
5. The Nomenclature of Steroids
Aede de Groot, Willem Koert
A steroid should get a correct name. For that reason the internationally agreed rules for the nomenclature of testosterone and other steroids will be presented here first.A correct nomenclature for steroids is of utmost importance for clear discussions. The best information about a compound can be collected from the structural formula but not everybody is well enough educated in chemistry to read and interpret structural formulas in a proper way. A correct name is then a good alternative. One has to know where one is talking about. People like to know which compound is the active principle in a medicine, a food supplement or an anabolic preparation.
For anabolic steroids four steroid skeletons with their own name are important for us; these are the gonane skeleton, the estrane skeleton, the androstane skeleton and the pregnane skeleton (see Figure 1).
The gonane skeleton is the most simple one, the C19 methyl group at C10 and the C18 methyl group at C13 are absent in this skeleton.
In the estrane skeleton only the methyl group at C10 is missing. This skeleton is also indicated as a 19-nor androstane skeleton, but that is not an official name. The prefix nor means that one C-atom is missing, in this case C19. One of the best known 19 nor steroids is nandrolone, which is also known as 19-nor-testosterone.
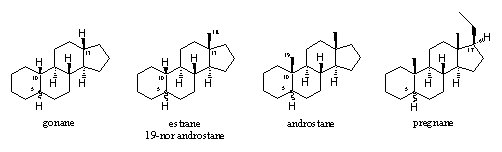
Figure 1
In the androstane skeleton both methyl groups at C10 and C13 are present
The pregnane skeleton has in addition also an ethyl group in the b-position at C17.
In all the four basic skeletons also the connection between the A- and B-rings, 5a or 5b has to be indicated. If both positions are possible for the H-atom at C5, or when its position is unknown, this is shown with a waving line in the formula or with the indication 5x in the name. When the H-atom at C5 is pointing to the bottom side this is indicated as 5a in the name and it will be drawn with a hatched bond in the formula. When this H-atom is pointing to the top side it is indicated as 5b in the name and drawn with a thick wedge shaped bond in the structural formula.
It is clear from the spatial structural formulas of the androstane skeleton in Figure 2, that it is important to know the position of this H-atom at C5, because the shape of the whole molecule in the region of the A-and B-rings is very different in both situations. The 5a skeleton is rather flat while the 5b skeleton shows a clear bend in the molecule. Since the shape of the steroid is important for its interaction with the receptor and thus for its biological activity, this is not something that should be neglected.
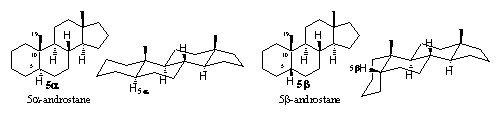
Figure 2
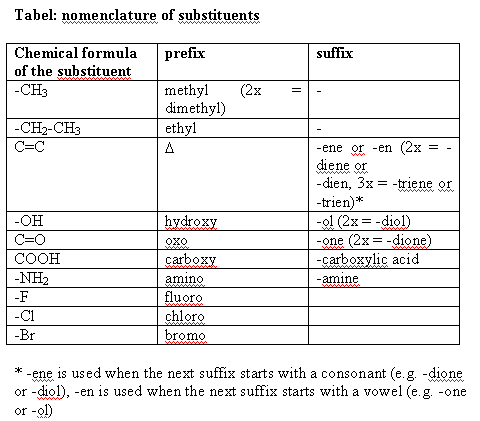
At all C-atoms of the steroid skeleton the H-atoms can be replaced by other atoms or groups of atoms. These atoms or groups are called substituents and each has its own name, place, direction and function. In chapter 2 we have already seen the methyl (-CH3) and ethyl (-CH2-CH3) substituents, which are derived from methane and ethane respectively. In the name of these substituents, the exit -ane is replaced by the exit -yl. In testosterone there are methyl groups at C10 and C13, in the pregnane skeleton there is an additional ethyl group at C17.
The substituent at C17 of the testosterone skeleton is a hydroxyl group or an alcohol group, or sometimes simply called an OH-group. In names it is indicated with the prefix hydroxy or with the suffix -ol. The substituent at C3 is called a carbonyl group or oxo group and in names it is indicated with the prefix oxo or with the suffix –one. A former name for this group is ketone, and this name has been maintained partly in the suffix.
A double bond between two C-atoms is mostly not considered as a substituent but a double bond has its own characteristic reactions and shape. The place of a double bond in a structural formula is indicated with the Greek capital D = D and in the name it gets the suffix –ene or -en. Steroid nomenclature using D = D is old-fashioned but it is still often met in the literature.
The suffixes –ene and –en are connected to the name of the skeleton and replace the suffix –ane. Thus gonane becomes gonene or gonen, estrane becomes estrene or estren, androstane becomes androstene or androsten, and pregnane becomes pregnene or pregnen The place of the double bond is indicated with a number before the name of the skeleton. The name of the steroid skeleton in testosterone is 4-androsten.
The most common substituents in anabolic steroids are collected in the table.
The position of the substituent in the steroid skeleton is indicated with the number of the C-atom, and its direction is indicated with the letters a and b. Both indications are placed before the name of the substituent.
Substituents are mentioned with a prefix before the name of the steroid skeleton or with a suffix behind the name. Just one suffix should be used in a name. When more substituents are present, as in testosterone and many other anabolics, these are mentioned as prefixes. These rules are not always applied in the correct way, but the following rules of thumb may help:
- Methyl and ethyl groups and the halogen atoms fluoro, chloro and bromo are placed before the name of the skeleton as prefixes.
- Hydroxyl groups can be indicated as the prefix hydroxy or as the suffix –ol.
- Double bonds are named as the suffixes –ene or –en, connected to the name of the skeleton (see above).
- Carbonyl groups are placed behind the name of the skeleton as the suffix -one.
In this book we will follow these conventions as strictly as possible.
|
Figure 3 |
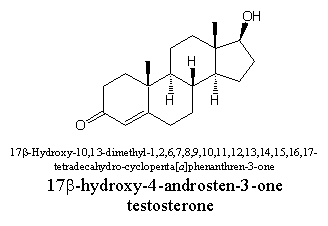
When all these rules, together with some that have not been discussed so far, are taken together, the official name of testosterone will become: 17b-hydroxy-10,13-dimethyl-1,2,6,7,8,9,10,11,12,13,14,15,16,17-tetradecahydrocyclopenta[a]phenanthren-3-one and that is of course an impossible name for every day use. Therefore some simplifications are made and four important steroid skeletons have got their own name as mentioned above.
Thus the steroid skeleton in testosterone is called an androstane skeleton, and the name 17b-hydroxy-4-androsten-3-one is already much simpler. It indicates that in the androstane skeleton of testosterone a hydroxyl group is pointing upwards at C17, that a double bond is located between C4 and C5 and that there is a carbonyl group at C3. When a compound is really important it usually has a so called trivial name for itself and in our case this trivial name is testosterone.
Nowadays computer programs are available that convert chemical names into structural formulas and visa versa. A good example of such a program is the chemical drawing program called Chem draw.
Chemists themselves have usually been among the first to create confusion in nomenclature, mostly for historical reasons. A compound like testosterone has been isolated for the first time from testes and is therefore called after that. Because the compound contains a steroid skeleton and a carbonyl group (a ketone group), the suffixes –ster and –one have been taken together and have been added. In this way the name testosterone is composed.
After some time more compounds with steroid skeletons are discovered and named in a similar way. When the number of compounds in the same group is large enough it becomes necessary to regulate their nomenclature internationally and after some or much discussion, rules are established. This is a time consuming process in which many authorities put forward their preferences. In the meantime several names have started to circulate, sometimes for the same compound.
Finally the IUPAC (Intenational Union of Pure and Applied Chemistry) takes a decision about the nomenclature for a class of compounds and after that these systematic names are used in the official chemical literature. However, the non official names are often firmly rooted and remain in use for years and years, especially in other sciences outside chemistry. Not seldom the official name is so complicated that nobody will use it anyhow and the historically established trivial name is maintained.
On the labels of bottles with pils and in publications about anabolic steroids, often the name etiocholane can be found. The prefix etio means degraded in Greek, so this name indicates a degraded cholane skeleton. This is an old-fashioned name for the 5b-androstane skeleton (see Figure 2), which originates from before the 2e world war.
The more common 5a-androstane skeleton is indicated in this nomenclature system with the name etioallocholane (see Figure 4). The Greek prefix allo indicates a small difference and is used also for other steroid skeletons with a 5a configuration.
Double bonds are mentioned as etiocholene and usually the double bond is located between C2 and C3. When the double bond is located at other positions, the prefix D is used in combination with the lowest number of the C-atoms on either side of the double bond.
Figure 4
After the 2e world war, an easier and straightforward nomenclature system has been adopted in 1950. This system is described above and generally used in the chemical literature [1]. Regularly updates of the nomenclature system have been published [2].
However in other sciences like biology, physiology, endocrinology, medicinal science and food science the old names are encountered often, although scientists have been urged regularly to use the correct steroid nomenclature [3].
In advertising and trade old fashioned names for anabolic steroids can be found every day. This confusing and misleading situation should come to an end as soon as possible. History learns however that this is not an easy task [3].
1. Steroid Nomenclature, Chemistry and Industry, London, (1950), 1-11;
Steroid Nomenclature, Journal of the Chemical Society, (1951), 3526.
2. Nomenclature of steroids, Pure and Applied Chemistry, (1972), vol. 1, 2, 285.
3. D.E. Kime, Steroid Nomenclature, General and Comparative Endocrinology, (1995), 98, 119.
|
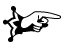
|