Het Anabolenboek
Willem Koert
Aede de Groot
Wageningen, 13/2/2007
8. The Mode of Action of the Steroid-Androgen Receptor Complex
Aede de Groot, Willem Koert
The formation of the steroid-androgen receptor complex is an essential step in all physiological processes, which are mediated by the androgen receptor. Before this complex can be formed the administered anabolic steroid or the natural testosterone has to be transported from the place where it is administered or biosynthesized to the cell where it has to find the androgen receptor.
The biosynthesis of testosterone takes place in the testes. The blood stream then takes care of the transport. This transport takes place in the free form or bound to transport proteins [1]. The binding to transport proteins is necessary because the free apolar testosterone is not well soluble in the water environment of the blood.
A second reason is to prevent early metabolic transformations of testosterone. When it is bound to proteins it is not anymore accessible for the enzymes that normally catalyze the transformation of testosterone itself (these reactions will be treated in the next chapters).
Only 2% of the testosterone is present in the blood in the free form, about 45% is weakly bound to serum albumin, a blood protein, and about 50% is tightly bound to the sex hormone binding globulin (SHBG). This SHBG also binds estradiol with approximately the same strength. The binding of testosterone to serum albumin is weak and a fast dissociation is possible. The relatively large portion of serum albumin bound testosterone can be mobilized quickly to the free form.
Only the free testosterone penetrates into the cells where it has to work. Cells have cell walls, but small molecules are able to pass. All kinds of (food) components also have to enter the cell continuously and all kinds of waste compounds have to be removed, so this should be possible.
The free steroids enter the cell from the blood stream through diffusion. This means that the spontaneous own mobility of the steroid is sufficient to bring it in the cell. There is no help of a transport protein necessary.
Once the steroid has arrived in the cell it has to find the androgen receptor. In the cell a large amount of different compounds is present, and together they make the enormous total of about 1014-1015 molecules, this is a number with 14-15 zero’s. These molecules are partly bound to cell membranes, but most of them move freely in the cell solution and continuous collisions occur between all these molecules. These collisions are very fast and at some time a collision will take place between a steroid molecule and the androgen receptor. Then recognition will take place based on the interactions that have been described in chapter 6.
The chance for a successful collision becomes larger when more steroid molecules and more androgen receptor molecules are present in the cell solution. In chemical words, favourable collisions become more probable at higher concentrations of steroid and receptor.
We now first will tell more about the androgen receptor and then proceed with the description of its fate and that of testosterone in the cell solution and in the cell nucleus. Several good reviews about this subject have appeared in the literature [2-6].
In the former chapter it has already been mentioned that the androgen receptor is a large protein, composed
from 919 amino acids. This chain of amino acids is folded in a unique way to a large loose ball. Inside and
outside of this ball several domains are distinguished, which all have their own function. For the androgen
receptor these domains are:
i) The Ligand Binding Domain (LBD), which takes care of the formation of the pocket and for the
binding of the ligands testosterone and dihydrotestosterone.
ii) The Nuclear Location Signal (NLS), which allows passing of the receptor-ligand complex through
the nuclear cell membrane into the nucleus.
iii) The DNA Binding Domain (DBD), which takes care of the binding of the receptor-ligand
complex to a specific element on the DNA that is called the Androgen Respons Element (ARE).
iv) The N-terminal Transactivation Domain (NTD), that helps to bind a number of assisting proteins.
These proteins are involved with the binding of the receptor-ligand complex to the ARE and with the copying of
the DNA that codes for the protein that has to be synthesised.
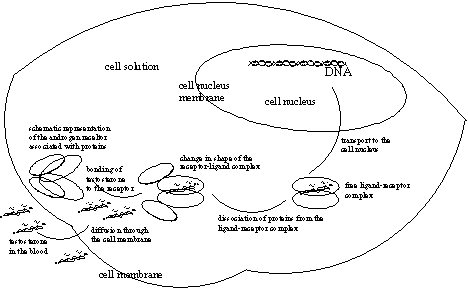
The empty androgen receptor is not present in the cell in a free form, but associated with other proteins. When testosterone or dihydrotestosterone binds in the pocket of the Ligand Binding Domain of the receptor a substantial change in shape of the complex takes place. This change is comparable with that of a fist closing around an object. (See Figure 1).
After this change of shape the receptor-ligand complex is set free from its associating proteins. This free new shape of the receptor-ligand complex makes new places available for new interactions.
Figure 1
Click for larger pic
One of these is the Nuclear Localisation Domain (NLS), which allows passing of the complex through the membrane that surrounds the nucleus of the cell (see Figure 1). The steroid can also pass the membrane around the nucleus of the cell on its own and then bind with androgen receptor that is located there.
A second free place takes care of dimerisation of the ligand receptor complex. Dimerisation is the going together of two molecules of ligand-receptor complex. All these associations and interactions are again based on the type of intermolecular forces that have been described in chapter 6, so on dipole-dipole interactions, hydrogen bonding and Van der Waals forces.
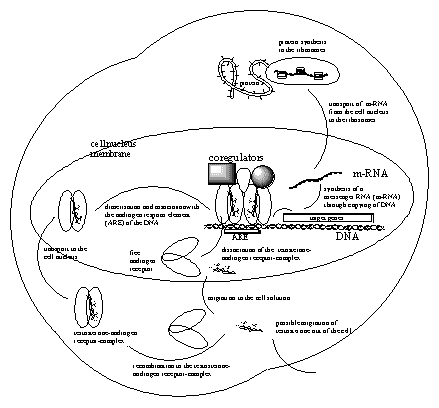
The ligand-androgen receptor dimer then will form a complex with the code element on the DNA. This code element is called the Androgen Respons Element (ARE), and its association with the receptor dimer is the signal for copying the gene that is carrying the code for the protein that has to be synthesized. (See Figure 2).
Figure 2
Click for larger pic
The copying of the correct stretch of DNA on the gene needs the help of enzymes. In the next chapter, more will be told about enzymes. Not only the copying itself needs help but also the whole process has to be regulated well. For that several coregulators and enzymes are brought together. (See Figuur 2).
One of these enzymes is an RNA polymerase and with the help of that enzyme the correct strecht of DNA on the gene is copied. In this way a so called messenger RNA (m-RNA) is biosynthesised, which contains a copy of the code on the DNA that codes for the protein that has to be synthesised.
This messenger RNA is transported out of the nucleus to the ribosomes in the cell solution where the actual protein synthesis takes place. In the ribosomes the code on the m-RNA is red and translated in the synthesis of a new protein. This new protein ultimately will carry out the task where the body is asking for. In the case of bodybuilders that is the biosynthesis of more muscle fibers.
In the cell solution and in the nucleus a continuous breakdown of proteins takes place. The biochemical term for this process is proteolysis, which means degradation of proteins. This degradation of proteins also takes place with the complex of enzymes and receptor on the DNA. After some time the whole construction falls apart and the ligand is set free again. This seems to be the fate of most of the steroid receptor complexes.
It is however also possible that the ligand-receptor complex dissociates again and that the whole process is ended in that way. This seems to be the case with the testosterone-androgen receptor complex. The free receptor and the testosterone migrate to the cell solution and may form again a complex to start a new cycle.
The free testosterone also may diffuse out of the cell back to the blood stream. In this way it can be transported to the liver where enzymes can transform it to an inactive derivative that can be excreted from the body. Such a transformation also may not take place and in that case the testosterone will enter the cell again and bind again to the androgen receptor. This phenomenon is called recirculation.
It will be clear that the processes, which are initiated by the testosterone-androgen receptor complex are based on a large number of delicate interactions. Everything is quite critical and it is not so easy to find new compounds which can stimulate all these processes better than the natural ligands. The association and dissociation processes have to take place on the right moment and to the right extend. A synthetic anabolic steroid will have a different shape and therefore a different fit and bonding in the pocket of the androgen receptor. This may ultimately cause a slightly different effect on the associations and dissociations of the different domains of the receptor.
An additional problem is the fact that the androgen receptor is present at many places in the body and it has to exercise different functions at different places. This probably will take place in each tissue in a slightly different way for each different ligand-receptor combination. In addition each individual is different. All these factors make it difficult to predict the effects and side effects of anabolic steroids and they will be different for each individual.
All these biological effects and side effects cannot be predicted directly from the structural formula of the anabolic steroid. This makes extensive testing of new anabolics very necessary. It is regrettable that this has not been carried out with many anabolics that appear in an uncontrolled way on the market. Users of such preparations take serious health risks and are the guinea pigs themselves.
[1] T. Saartok, E. Dahlberg and J.A. Gustafson,
Endocrinology (1984) 114, 2100-2106.
[2] E.T. Keller, W.B. Ershler and
C. Chang, Frontiers in Bioscience (1996) 1, d59-71.
[3] J. Gobinet, N.
Pujol en Ch. Sultan, Molecular and Cellular Endocrinology (2002) 198,
15-24.
[4] H.-J. Lee and C.Chang, Cellular and Molecular Life Sciences
(2003) 60, 1613-1622.
[5] D.K. Lee and C. Chang, The Journal of Clinical
Endocrinology and Metabolism (2003) 88, 4043-4054.
[6] W. Gao, C.E Bohl
and J.T. Dalton, Chemical Reviews (2005) 105, 3352-3370.
|
|