Het Anabolenboek
Willem Koert
Aede de Groot
Wageningen, 5/3/2007
9. How Do Enzymes Work?
Aede de Groot, Willem Koert
Enzymes are involved in practically all transformations of substances in Nature. They help and control all chemical reactions of carbon compounds in our body. In the next chapters we will see how enzymes are involved in conversions of anabolic steroids. In chapter 11 the biosynthesis of the male (testosterone and dihydrotestosterone) and the female (estrone and estradiol) sex hormones will be discussed. We there will see how enzymes help in the biosynthesis of these compounds.
The word biosynthesis indicates the way carbon compounds are made in Nature by living organisms. The same carbon compounds mostly can be prepared also in laboratories, this process is simply called synthesis, chemical synthesis or organic synthesis.
Biosynthesis is part of the metabolism of carbon compounds. Metabolism is the combination of the synthesis of carbon compounds, the anabolism, and the degradation of carbon compounds, the catabolism. In Nature a continuous synthesis and degradation of carbon compounds takes place and this dynamic situation is called life.
All reactions in living organisms in Nature need the help of enzymes. Enzymes are proteins which catalyze (help) and control chemical reactions. There are only a few reactions in Nature which do not need an enzyme for help and proceed spontaneously, and that is a good thing. Spontaneous reactions in Nature are not welcome, because they run out of control easily and are sometimes difficult to redress. An example may be the burning of fat. At high temperature this is a spontaneous reaction which can be controlled only be the effluent of fuel.
In our body we also produce part of our energy by the burning of fat. This can of course not take place at high temperature, our body temperature has to be maintained at about 37oC. Therefore the burning of fat has to take place in a controlled way. It is however also not so easy to burn fat at the low temperature of 37oC and this process needs help. Both functions, the control and the help, are carried out by enzymes.
Enzymes are involved in all anabolic and catabolic reactions of organic compounds in our body. In chemical words we say that enzymes catalyse all chemical reactions in our body. Enzymes do this by forming a complex with the compound, called the substrate, which they have to transform. The formation of this so called enzyme-substrate complex can be compared with the formation of a ligand-receptor complex. The same interactions are involved, only the purpose of the complex formation is different.
The enzyme has to transform the substrate into another compound, or in other words, the substrate has to undergo a chemical reaction. The purpose of a ligand-receptor complex is a change of the shape of the receptor. The ligand does not undergo a transformation and leaves the receptor unchanged when it has fulfilled its task.
The formation of an enzyme-substrate complex and the subsequent reaction of the substrate to another compound is more complicated than just the formation of a complex. Mostly a second compound has to be brought into contact with the enzyme-substrate complex before the reaction can take place. This second substance can be a simple compound like water, which is brought in contact with the substrate by the enzyme. The reaction of a substrate with water is called a hydrolysis.
After the reaction the hydrolysis products leave the enzyme again. This is usually not difficult because the separate hydrolysis products have a weaker interaction with the enzyme than the original substrate. After the products have left the enzyme a new substrate and a new water molecule can enter the enzyme pocket and the next hydrolysis can take place. The enzyme itself does not change during the reactions, it only helps to bring the substrate and the water together thus stimulating the reaction.
Enzymatic reactions are mostly equilibrium reactions, this means that the reaction can proceed in two directions. Equilibria are indicated in the reaction scheme with a double arrow as is shown below. The names of enzymes usually have the suffix -ase, which is added to the kind of reaction they catalyse. The general name of enzymes which catalyse hydrolysis is therefore hydrolase.
Hydrolysis reactions only need water as a second compound for the reaction, but many reactions need more complicated compounds for their transformations. These more complicated assisting substances are generally known as co-enzymes. They are more complicated then water but they are not proteins and much simpler than the enzyme itself. The substrate and the co-enzyme are again brought together by the enzyme and then the desired reaction can take place. Usually the co-enzyme is regenerated after the reaction.
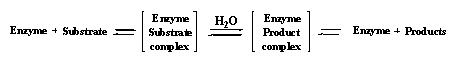
Parts of these co-enzymes we have to consume with our food and they are better known as vitamins. These compounds were recognized long before as being of vital importance. We now know that they help enzymes to perform their reactions and to keep our metabolism going. We will not go deeper into the role and working mechanisms of co-enzymes, but concentrate on reactions that are important for the metabolism of anabolic steroids.
It is known that anabolic steroids undergo many enzymatic transformations in the body. Mostly they are undesired because they transform the anabolic in other inactive compounds before it can perform its duty.
In this respect, oxidations and reductions are important metabolic processes for steroids. For that reason it is good mention here the chemical definition of these reactions. This is essential for all enzymes which catalyse oxidation and reduction reactions. These enzymes are known under the general names cytochrome P450 enzymes, oxidoreductases (dehydrogenases), reductases and aromatase.
Oxidation is the introduction of oxygen (O) in a compound or the removal of hydrogen (H) from a compound. In normal life the reaction of a compound with oxygen is also termed as combustion.
Reduction is the opposite of oxidation. It involves the removal of oxygen from a compound or the introduction of hydrogen into a compound.
|
|