09.11.2005
Cobalt chloride administration in athletes: a new perspective in blood doping?
We have recently hypothesised that gene therapy targeting the hypoxia inducible factor (HIF) pathway may be an attractive alternative to traditional techniques of blood doping.
Hypoxia activates a large number of genes that have essential roles in cell and tissue adaptation to conditions of low oxygen. Such a complex response is mainly mediated through endogenous gene modulation at the HIF pathway.
Cobalt is a relatively rare transition metal with properties similar to those of iron, chromium, and nickel. Cobalt chloride, a water soluble compound traditionally used to treat anaemia in pregnant women, infants, and patients with chronic anaemia undergoing long term haemodialysis, is a well established chemical inducer of hypoxia-like responses, such as erythropoiesis and angiogenesis in vivo.
The precise mechanism of this induction is not fully understood. However, the hypoxialike response probably involves increased DNA binding activity of HIF1a, as cobalt stabilizes HIF1a trough generation of reactive oxygen species by a non-enzymatic, nonmitochondrial mechanism. The final result of this induction is enhanced erythropoietin production and more efficient stimulation of the erythropoietic response, achievable at the moderate oral dose of 30 mg/kg.
Besides the relevant therapeutic benefits, especially in patients with acute myocardial ischaemia, cobalt chloride administration may have alternative and obscure potential applications. Given the athlete’s innate inclination to experiment with novel doping strategies, irrespective of the biological and health risks, this low cost, water soluble compound may be an attractive, less challenging, and equally effective means of boosting endogenous erythropoietin production.
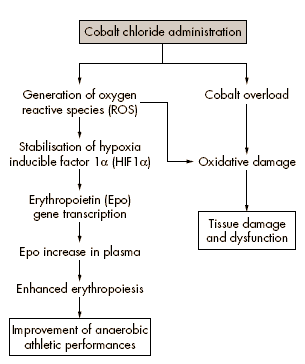
Cobalt supplementation is not banned, and thus virtually undetectable by current anti-doping testing for blood doping. Objective and definitive information on absorption, disposition, and health risks of inorganic cobalt salts after oral administration is not as yet available.
Although several studies have reported no mortality and no appreciable toxic effects of cobalt chloride, even at the high oral dose of 40 mg/kg for up to four months, cobalt overload was earlier identified as a contributing factor to alcohol related cardiomyopathy.
In addition, it has been reported that liver, kidney, and heart accumulates cobalt to a greater extent, causing hepatotoxicity, nephrotoxicity, organ damage and dysfunction, even at a dose of 33.3 mg/kg. Oxidative stress is often cited as a possible cause.
Excessive administration of this trace element also produces goitre and reduced thyroid activity.
Bron:
Br J Sports Med. 2005 Nov;39(11):872-3.